Zihe Zheng
Identify Obesity and Adiposity-Related CKD Subgroups and Metabolites: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study
Abstract
BACKGROUND
Obesity/adiposity perturbs the plasma metabolome, as does chronic kidney disease (CKD). Understanding the complex relationships across CKD patient sub-phenotypes, obesity, and the metabolome may shed light on finding novel risk factors and the mechanisms for CKD progression.
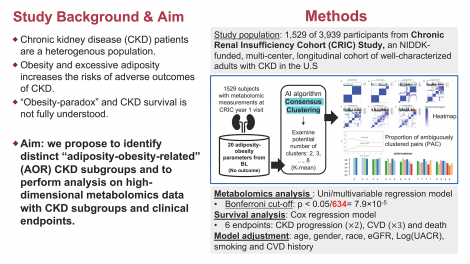
METHODS
Among 1,529 participants in the CRIC Study for whom metabolomics data (Broad Institute) were generated, we first applied consensus clustering with K-means on 20 baseline clinical adiposity-obesity-related attributes to identify patient subgroups. We individually examined the association of 634 known metabolites with the identified subgroups using separate multivariable linear models. Finally, we used Cox model to examine the prospective associations of the adiposity-obesity subgroups with six clinical endpoints.
RESULTS
We identified three distinct adiposity-obesity-related CKD subgroups: Subgroup 1 (N=708) with relatively lower prevalence of diabetes and preferable obesity profiles, Subgroup 2 (N=464) with relatively high prevalence of uncontrolled diabetes, and Subgroup 3 (N=357) with low HDL level, relatively high prevalence of diabetes, and less preferable obesity profiles. Among the 634 known metabolites, 179 were significantly associated with CKD subgroups at Bonferroni-adjusted p<7.9×10-5. Survival analyses showed that compared to Subgroup 1, Subgroup 2 had the highest risk for CKD progression and ESKD, while Subgroup 3 had the highest risk for the composite CVD outcome.
CONCLUSION
With consensus clustering and metabolomics analysis, we discovered three distinct adiposity-obesity-related subgroups of CKD patients that were associated with numerous metabolites and different risks of clinical endpoints. Novel biomarkers that co-segregate with different patient subgroups could reveal new insights into the obesity related biology of CKD.
Keywords
Patients heterogeneity, CKD, obesity, adiposity, clustering analysis, metabolomics, survival analysis, ESKD, CVD, deathCommenting is now closed.
About Us
To understand health and disease today, we need new thinking and novel science —the kind we create when multiple disciplines work together from the ground up. That is why this department has put forward a bold vision in population-health science: a single academic home for biostatistics, epidemiology and informatics.
© 2023 Trustees of the University of Pennsylvania. All rights reserved.. | Disclaimer



Comments