Jessie Torgersen
Risk of Acute Liver Injury Associated with Protease Inhibitor-Based Direct-Acting Antiviral Therapy for Hepatitis C
Abstract
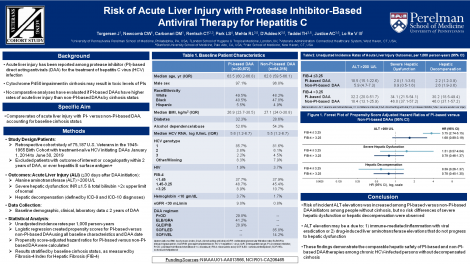
Cases of acute liver injury (ALI) have been reported among chronic hepatitis C virus-infected initiators of protease inhibitor (PI)-based direct-acting antiviral (DAA) regimens, predominately with decompensated cirrhosis. No comparative analyses have evaluated whether initiators of PI versus non-PI-based DAAs have higher risk of ALI events, stratified by advanced hepatic fibrosis/cirrhosis status.
We conducted a cohort study including 20,872 initiators of PI-based (paritaprevir/ritonavir/ombitasvir +/- dasabuvir, elbasvir/grazoprevir, glecaprevir/pibrentasvir) and 54,315 initiators of non-PI-based DAA therapies (sofosbuvir/ledipasvir, sofosbuvir/velpatasvir) in the 1945-1965 Veterans Birth Cohort (2014-2019). We determined development of: 1) alanine aminotransferase (ALT) >200 U/L, 2) severe hepatic dysfunction (coagulopathy with hyperbilirubinemia), and 3) hepatic decompensation, all within 12 months of DAA initiation. Cox regression was used to determine propensity score-adjusted hazard ratios (HRs) with 95% confidence intervals of each outcome in PI versus non-PI initiators, stratified by baseline advanced hepatic fibrosis/cirrhosis status by FIB-4.
Among persons with baseline FIB-4 ≤3.25, PI initiators had higher risk of ALT >200 U/L (HR, 3.75 [2.74-5.15]), but not severe hepatic dysfunction (HR, 1.51 [0.57-4.04]) or hepatic decompensation (HR, 0.59 [0.26-1.37]), compared to non-PI-initiators. For those with baseline FIB-4 >3.25, PI initiators did not have significantly higher risk of ALT >200 U/L (HR, 1.68 [0.89-3.19]), severe hepatic dysfunction (HR, 0.79 [0.46-1.37]), or hepatic decompensation (HR, 0.78 [0.45-1.35]) than non-PI initiators.
While risk of incident ALT elevations was increased among PI-based DAA initiators with FIB-4 ≤3.25, risk of severe hepatic dysfunction and hepatic decompensation did not differ between PI and non-PI-based DAA initiators in either FIB-4 stratum.
Keywords
acute liver injury, protease inhibitor, direct-acting antivirals, hepatitis CCommenting is now closed.
About Us
To understand health and disease today, we need new thinking and novel science —the kind we create when multiple disciplines work together from the ground up. That is why this department has put forward a bold vision in population-health science: a single academic home for biostatistics, epidemiology and informatics.
© 2023 Trustees of the University of Pennsylvania. All rights reserved.. | Disclaimer